What is E 171 additive?
Food additive E 171 titanium dioxide is an inorganic, insoluble, white artificial colouring with very good stability to light, heat, oxidation and pH changes.
Synonyms White pigment C16.
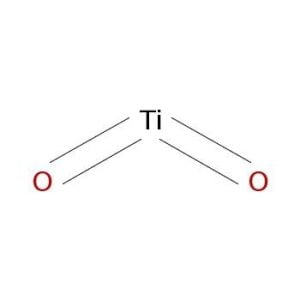
The rough formula is: TiO 2
Molecular mass is M=79,88
Its chemical name is titanium dioxide.
In nature it exists in the form of three crystalline modifications:
– rutile,
– anatas and
– brookit.
The rutile and the anastasis have a quadratic lattice and the bookit has a rhombic lattice.[i]
Titanium dioxide consists mainly of pure titanium dioxide in the form of anatase and/or rutile which may be coated with small amounts of alumina and/or silica to improve the technological properties of the product.
How is titanium dioxide obtained?
Titanium dioxide can be obtained by cold or hot hydrolysis of TiCl solutions4 , which occurs in the presence of SO42- , PO43- ions or an excess of Cl– or NO3 ions, as well as by direct oxygen oxidation of TiCl4 in the vapour phase.
It can also be obtained by hot hydrolysis of titanium or TiO2 XH2 O sulphide solutions by dehydration and heating to 700o C (currently NaF, borate or barium phosphate as mineraliser).
Hydrated titanium dioxide, obtained by such methods, is transformed by calcination and subsequent treatment into the product used as pigment. It is presented as a white amorphous powder. It is insoluble in water, organic solvents and alkali hydroxides, but is readily soluble in hydrofluoric acid, hot concentrated sulphuric acid and in the melts of alkali hydroxides or carbonates.
By heating the TiO2 powder to 250-6000 C in an oxidizing atmosphere, a yellow-brown to greenish-brown coloration occurs, but disappears on cooling.
At temperatures above 29270 C it decomposes into T i2 O3 and O2 .
TiO2 and O2 has a density of 3.6-3.95 g/cm3 for the anatase form and 4.1-4.4 g/cm3 for the brookite and rutile forms.
Melts at 1855o C. The loss on drying of the additive at 1050 C for 3 hours is maximum 0,5% and on burning at 800 o C maximum 1% relative to the volatile-free product.1
Certain rutile forms of titanium dioxide are produced using mica (also known as potassium aluminium silicate) as a template to form the basic granular structure. Mica is surface coated with titanium dioxide using a specialised patented process. Titanium dioxide rutile in granular form is manufactured by subjecting the pearlescent pigment consisting of mica coated with titanium dioxide (rutile) to an extractive dissolution in an acid medium followed by an extractive dissolution in alkali. By this process all mica is removed and the resulting product is a granular form of rutile titanium dioxide.[ii]
Which foods contain the food additive E 171?
Titanium dioxide is used as a food colour (E171), its technological function is to make food more visually attractive, to give colour to otherwise colourless food or to restore the original appearance of food.
Food colour E 171 is used in:
– candy making,
– matt white finish or as a base for other colours,
– colouring of some cheeses,
– chewing gum manufacture,
– chocolate and ice cream making,
– making creams, soups, sauces (for infants, young children and adolescents)
– manufacture of bakery products, cakes or other pastry products,
– manufacture of tomato paste type broth,
– processed fish and fishery products, including molluscs and crustaceans,
– unprocessed fish,
– unprocessed molluscs and crustaceans,
-production of fruit and vegetable preparations (except compotes),
– manufacture of food supplements, etc.
It is available in both water- and oil-dispersible forms, as suspensions in water, glycerine, propylene glycol, glucose syrups, vegetable oils. [iii], [iv],[v]
What else can E 171 be used for?
E 171, titanium dioxide is approved as an ingredient in food, pharmaceuticals and cosmetics. Titanium dioxide, which is not in nanoparticle form, is not absorbed on the skin. It acts as a physical UV protective barrier.
It increases the opacity of other pigments in make-up products and improves their adhesion to the skin. Reduces the transparency of cosmetic compositions.[vi]
Titanium dioxide as a white pigment is used in the manufacture of paints, varnishes, adhesives, plastics, rubber, textiles, ceramics, white cement, roofing materials, white cement, floor coatings, paper and printing inks.
Used as white pigment it must have a high purity. Its opacity and brilliance come from its ability to scatter light. It is brighter than diamond. Only rutile and anatase have good pigmenting properties.
In the textile industry it is used in yarn guides so that the yarns slide smoothly during spinning.[vii]
What are the characteristics of E171 titanium dioxide?
The table below shows the specifications set for titanium dioxide by Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council.
| TITANIUM DIOXIDE | E 171 |
| Composition | Content not less than 99 % on the alumina- and silica-free matter |
| Description | White to slightly coloured powder Identification Solubility Insoluble in water and organic solvents. |
| Solubility | Dissolve slowly in hydrofluoric acid and hot concentrated sulphuric acid. |
| Purity | |
| Loss on drying | Not more than 0,5 % (105 °C, 3 hours) |
| Loss on ignition | Not more than 1 % on a volatile matter-free basis (800 °C) |
| Aluminium oxide and/or silicon dioxide | In total not more than 2.0%. |
| Substances soluble in 0,5 N HCl | Not more than 0,5 % on an alumina- and silica-free basis and, in addition, for products containing alumina and/or silica, not more than 1,5 % on the product as sold. |
| Water-soluble substances | Not more than 0,5 % Cadmium |
| Cadmium | Not more than 1 mg/kg after extraction with 0,5 N HCl |
| Antimony | Not more than 2 mg/kg after extraction with 0,5 N HCl. |
| Arsen | Not more than 1 mg/kg after extraction with 0,5 N HCl. |
| Plumb | Not more than 10 mg/kg after extraction with 0,5 N HCl |
| Mercury | Not more than 1 mg/kg after extraction with 0,5 N HCl |
Are there any side effects from consuming the food additive E 171?
After conducting a review of all available relevant scientific evidence, EFSA concluded that a genotoxicity concern for TiO particles cannot be excluded2 . Based on this concern, EFSA experts no longer consider titanium dioxide safe when used as a food additive. This means that an acceptable daily intake (ADI) for E171 cannot be established.
France was the first country in Europe to decide to suspend this additive.
In March 2021 the EFSA Panel concluded that although gastrointestinal absorption of TiO2 particles is low, they can accumulate in the body. General and organ toxicity studies showed no adverse effects with E 171 up to a dose of 1,000 mg/kg body weight/day, nor with TiO2 NPs (> 30 nm) up to the highest dose tested of 100 mg/kg body weight/day. No reproductive and developmental toxic effects were observed up to a dose of 1,000 mg E 171/kg body weight/day, the highest dose tested in the EOGRT study. Regarding genotoxicity, the group concluded that TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not genetic mutations. No clear correlation was observed between the physicochemical properties of TiO2 particles and the outcome of genotoxicity tests either in vitro or in vivo.
A limit value for TiO2 particle size could not be identified for genotoxicity. No studies adequately designed to investigate the potential carcinogenic effects of TiO2 were available.
EFSA’s assessment relates to the risks of TiO2 used as a food additive, not to other uses.
A study on the oral systemic availability of the additive E 171 was conducted by Pele et al. (2015) on 8 volunteers (7 completed the study). Study participants ingested two capsules containing 50 mg of E 171 with 250 ml of water. Blood samples were taken at 0, 30 min and 1, 1.5, 2, 3, 6, 8 and 10 h after ingestion. Tea and coffee with milk and/or sugar were allowed from 2 hours after ingestion. Blood droplets were spread on a glass slide and protected from drying. Particles were detected by light microscopy at 100-fold magnification and then 400-fold magnification using a dark field condenser. This method could only be performed in samples from five of the seven subjects due to blood clotting in samples from two of the subjects. The degree of reflectance (0, 1, 2 and 3) was used as a semi-quantitative measurement of the number of TiO particles2 .
The total blood Ti concentration was measured. The authors observed that there are two absorption pathways for particles in the human intestine, one in the duodenum/jejunum and one in the colon. The highest blood Ti concentration determined in any study participant was approximately 11 ng/mL (at 6 hours). Thereafter, the blood Ti concentration decreased to about 5 ng/mL at 10 hours after administration. The researchers concluded that after oral administration of 100 mg E 171 to volunteers (humans) the blood Ti concentration and particle number increased, demonstrating the oral systemic availability of TiO2.[viii]
Conclusions and Legislative Regulations E 171
Titanium dioxide (E 171) was originally authorised as a food additive in the EU under Annex II of Regulation (EC) No 1333/2008.
The safety of the food additive E 171 was re-evaluated by the EFSA Panel ANS in 2016 under Regulation (EU) No 257/2010 as part of the re-evaluation programme of food additives authorised in the EU before 20 January 2009.[ix]
The European Commission (EC) adopted on Friday, 14 January 2021, a ban on the use of titanium dioxide (E171) as a food additive.
The Commission proposal is based on a scientific opinion of the European Food Safety Authority, which concluded that E 171 can no longer be considered safe when used as a food additive, in particular because concerns about genotoxicity cannot be excluded.
The ban on the use of E 171 will apply after a transitional period of six months. Therefore, from summer 2022, this additive should no longer be added to food.[x]
Author: dr.ing. Ancuta Fulvia Manolache
Bibliographical references
[i] Elena Orănescu, Food Additives-necessity and risk, SemnE Publishing House, 2005, Bucharest
[ii] https://op.europa.eu/ro/publication-detail/-/publication/a42dd9b2-b63f-438b-a790-1fa5995b7d41#:~:text=Regulamentul%20%28UE%29%20nr.%20231%2F2012%20al%20Comisiei%20din%209,cu%20relevan%C8%9B%C4%83%20pentru%20SEE%29%20Desc%C4%83rc%C4%83ri%20%C8%99i%20versiuni%20lingvisticeClose?msclkid=f6f4375eb00911ec85eadd08929ec1c4
[iii] Raluca Stan, Natural and synthetic food additives, 2007, Printech Publishing House, Bucharest, Romania
[iv]https://www.efsa.europa.eu/en/news/titanium-dioxide-e171-no-longer-considered-safe-when-used-food-additive
[v] https://eur-lex.europa.eu/legal-content/RO/TXT/PDF/?uri=CELEX:02008R1333-20160525&from=FR
[vi] http://www.chemicalsystem.ro/ro/prod/dioxid-de-titan/materii-prime-chimice-77/
[vii] https://ro.spreckelsunionsd.org/rutile-487
[viii] https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2021.6585
[ix]https://www.efsa.europa.eu/en/news/titanium-dioxide-e171-no-longer-considered-safe-when-used-food-additive
[x]https://www.efsa.europa.eu/en/news/titanium-dioxide-e171-no-longer-considered-safe-when-used-food-additive
13https://pixabay.com/ro/photos/dulciuri-bomboane-bomboane-albe-7070041/
14https://pixabay.com/ro/photos/valentine-s-day-valentine-cookie-uri-3984154/