What is potassium phosphate E340?
Potassium phosphate (E340) is a food additive belonging to the class of acidifying, sequestering, stabilising, softening, buffering and neutralising agents. Antioxidant of inorganic nature.
What forms does potassium phosphate come in?
Potassium phosphate comes in three forms:
- E 340 (I) – Monopotassium phosphate;
- E 340 (II) – Dipotassium phosphate;
- E 340 (III) – Tripotassium phosphate.
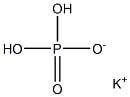 The additive E 340 I has the following synonyms: monobasic potassium phosphate, monipotassium monophosphate, acid potassium phosphate, potassium orthophosphate. Its chemical name is potassium dihydrogen phosphate, mono potassium dihydrogen orthophosphate or mono potassium dihydrogen monophosphate. Gross formula: KH2 PO4 , and molecular mass, M = = =136,09 g/mol.
The additive E 340 I has the following synonyms: monobasic potassium phosphate, monipotassium monophosphate, acid potassium phosphate, potassium orthophosphate. Its chemical name is potassium dihydrogen phosphate, mono potassium dihydrogen orthophosphate or mono potassium dihydrogen monophosphate. Gross formula: KH2 PO4 , and molecular mass, M = = =136,09 g/mol.
Industrial is obtained by mixing an appropriate amount of phosphoric acid with potassium hydroxide or potassium carbonate solution.[i]
It is found as colourless, tetragonal crystals, crystalline powder or white granules. It is odourless and hygroscopic.
It is slightly soluble in water (33g per 100 ml water at 250 C) with hydrolysis, but insoluble in ethanol.
The melting point is 2530 C and the pH of a 1% aqueous solution is between 4.2-4.8. In the presence of air it is stable, but on heating to 4000 C it transforms first to pyrophosphate and then to metaphosphate. 1, [ii]
 Additive 340 II has the following synonyms: dipotassium monophosphate, secondary potassium phosphate, dipotassium acid phosphate, dipotassium orthophosphate, dibasic potassium phosphate. Its chemical name is dipotassium hydrogen monophosphate, dipotassium hydrogen phosphate, dipotassium hydrogen orthophosphate.
Additive 340 II has the following synonyms: dipotassium monophosphate, secondary potassium phosphate, dipotassium acid phosphate, dipotassium orthophosphate, dibasic potassium phosphate. Its chemical name is dipotassium hydrogen monophosphate, dipotassium hydrogen phosphate, dipotassium hydrogen orthophosphate.
Its gross formula is K2 HPO4 and its molecular mass is M=174.18 g/mol.
Industrial is obtained by treating phosphoric acid with potassium carbonate or potassium hydroxide. Dipotassium orthophosphate occurs as white crystals or colourless paste, as well as white crystals or white powder.
It is a very hygroscopic and even deliquescent substance (it absorbs water vapour from the air to form a solution).
It is soluble in water (89g/100ml water) and the pH of its 1% aqueous solution is between 8.7-9.4.
It is insoluble in ethanol and is converted to pyrophosphate by calcination.
The food additive must contain not less than 98% active substance after drying at 105°C for four hours and the loss on dehydration must not exceed 2%. [iii]
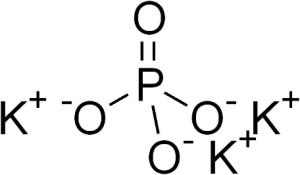 The additive E340 III has the following synonyms: tribasic potassium phosphate, tripotassium orthophosphate and its chemical name is tripotassium monophosphate, tripotassium phosphate, tripotassium orthophosphate. It is found in the anhydrous state K3 PO4 with molecular mass M=212,27 or hydrated with n molecules of water K3 PO4 .nH2 O, where n=1 or 3.Industrial is obtained by treating phosphoric acid with excess potassium hydroxide.
The additive E340 III has the following synonyms: tribasic potassium phosphate, tripotassium orthophosphate and its chemical name is tripotassium monophosphate, tripotassium phosphate, tripotassium orthophosphate. It is found in the anhydrous state K3 PO4 with molecular mass M=212,27 or hydrated with n molecules of water K3 PO4 .nH2 O, where n=1 or 3.Industrial is obtained by treating phosphoric acid with excess potassium hydroxide.
It occurs as colourless or white, odourless, hygroscopic crystals or granules. Available hydrated forms include monohydrate and trihydrate.
It is soluble in water but insoluble in ethanol.
It hydrolyses strongly, the solution having an alkaline character. The pH of a 1% aqueous solution is between 11.5 and 12.3.
It is a very hygroscopic even deliquescent odorless substance.
It must contain not less than 97 % K3 PO4 calculated on the calcined substance (8000 C for 30 minutes) and not more than 0,2 % insoluble substances on the dry substance. It also contains between 30,5 % and 34,0 % P2 O5 on the ignited basis. 1, [iv], [v]
Need for use of E340 (I, II, III) additives
E340 (I, II, III) additives are used in foods for their role as:
– buffering, neutralising agents: as buffering substances, the mixture in various proportions of monopotassium and dipotassium orthophosphate maintains the pH constant of the medium in which it is incorporated.
– sequestering agents: they bind heavy metals in some foodstuffs to form complex combinations (chelates), which inhibit the catalytic action of these elements;
By sequestering metal ions (copper, iron, lead), their prooxidant action is prevented, thus inhibiting the rancidity reaction of fats (oil, butter, etc.), prolonging their shelf life, preserving the flavour of products containing fats or emulsified oils and preventing the appearance of rancid taste and smell (in confectionery and pastry products). The unaltered flavour of butter or margarine is also preserved.
– Antioxidants: E340 additives have a synergistic role when used together with antioxidants (BHA, BHT, propyl gallate, ascorbic acid, isoascorbic acid).
– stabilisers, etc.
Dipotassium and tripotassium orthophosphates in some foods act as acid neutralisers (have a basic pH).
E 340 additives solubilise, emulsify and stabilise proteins (in processed cheese).
Which foods contain E340 (I, II, III) food additives? Maximum permitted use limits.
Current legislation requires that the use of E340 (I, II, III) additives be used separately or in a mixture with E 339 (I, II, III), E341 (I, II, III), E343, E450, E451 and E452 in the following foodstuffs:
- in non-alcoholic flavoured drinks at a dose of 700mg/l;
- in energy drinks and prepared water 0.5g/l;
- in vegetable protein drinks 20g/l;
- in alcoholic beverages (excluding wine and beer) 1g/l;
- in coffee-based drinks for vending machines 2g/l;
- in sterilised milk based on UHT 1g/l;
- in partially dehydrated milk with a content of up to28%s.u. 1g/l;
- in partially dehydrated milk with a content of more than 28% s.u. 1,5g/l;
- in skimmed milk powder 2,5 g/kg;
- in pasteurised, sterilised (UHT) cream 5g/kg;
- cream and vegetable fats 5g/kg;
- in processed cheese and processed cheese-like products 20g/kg;
- in fresh cheese (excluding Mozzarella) 2g/kg;
- in milk-based chocolate and malt beverages 2g/l;
- in butter and sour cream preparations 2g/kg;
- in meat products is used in doses of 5g/kg;
- in unprocessed, chilled or frozen fish fillets 5g/kg;
- in unprocessed or processed, chilled, frozen molluscs and crustaceans 5g/kg
- in canned shellfish products 1g/kg;
- in fish and shellfish paste and fat spreads (excluding butter) in cans 5g/kg
- confectionery 5g/kg, in icing sugar 10g/kg, in flavoured syrups for ice cream and similar products 3g/kg,
- in chewing gum “qs”, mixtures and dessert powders 7g/kg, in desserts 3g/kg, in candied fruit as well as in fruit preparations 800mg/kg
- in processed potato products and prepared, chilled, frozen potatoes 5g/kg
- in snacks 1,5 g/kg
- in liquid egg 10g/kg
- in tea and herbal infusion 2g/kg,
- in cider and pear brandy 2g/l.
- can be added in dietary supplements “qs”, in salt and its substitutes 10g/kg
- in plant washing agents 30g/kg or dispensers 50g/kg
- in food ice 1g/kg
- in bakery and pastry products, additives are used for fine pastry products 20g/kg;
- in flour 2,5 g/kg
- in flour with flour conditioners 20g/kg
- in doughs 5g/kg
- in noodles 2g/kg as well as in aqueous emulsions for coating pastry moulds
- in sauces 5 g/kg
- in soups and broths 3 g/kg
- in chewing gum “qs”
- in powdered foodstuffs 10 g/kg
- in breakfast cereals 5 g/kg
- in snacks 5 g/kg [vi]
- in dietary foods for infants for special medical purposes and special infant formula 100mg/kg
- in processed cereal-based foods and foods for infants and young children, the maximum level of phosphates when used as – Food additives other than carriers in food additives is 40 000 mg/kg individually or in combination in the preparation (expressed as P2 O5 ) and may be used in colour preparations.
- in food additives, including carriers, in food enzymes, the maximum level to which potassium phosphates may be added in the enzyme preparation is 50 000 mg/kg (individually or in combination expressed as P2 O5 ). [vii]
Are there any side effects from consuming E340 food additives?
Phosphates are essential constituents of the human body, which is why it is necessary that the food consumed also contains these substances. The phosphate content of the blood is kept constant due to constant exchange with the mineral substances of the skeleton. This exchange is directed by the parathyroid hormone. The calcium ion in the blood plays an important role because it regulates the amount of hormone entering the blood. Some experiments on rats have shown that when the diet is high in phosphates, they suffer parathyroid hypertrophy, kidney stones and calcification of the aorta.
Other experiments have shown that too much phosphate intake can prevent the assimilation of other minerals (in the long term it can reduce the body’s natural calcium and phosphorus balance). It has also been observed that higher intake causes hyperactivity and digestive problems. 1, [viii]
The toxicity of phosphates was first evaluated by the EU Scientific Committee for Food and then by JECFA (Joint FAO/WHO Expert Committee on Food Additives). It concluded that setting an Acceptable Daily Intake (ADI) of 30 mg/kg body weight was not appropriate for phosphates “because phosphorus is an essential nutrient and an unavoidable constituent of food” and therefore it was decided to set a “Tolerable Maximum Daily Intake” (MTDI) rather than an ADI.
The MTDI established was 70 mg/kg body/day (expressed as phosphorus) for the mixture of phosphates and polyphosphates, both naturally occurring in food and ingested as food additives. [ix][x]
Conclusions and Legislative Regulations – E340
Potassium phosphates are regulated at European level by Regulation (EU) No 1129/2011[xi] and at national level there is a regulation (12 July 2002) on food additives for use in foodstuffs for human consumption, which sets maximum permitted limits for each category of food product where they can be used.6
The additives E340 (I, II, III), potassium phosphates, can be found in many food products so it is very important to carefully count the amount of phosphorus ingested during a day so as not to exceed the recommended dose.
Author: dr. ing. Fulvia Ancuța Manolache
Bibliographical references
[i] Elena Olanescu, Food Additives-necessity and risk, SemnE Publishing House, 2005, Bucharest, pp. 164-168;
[ii] https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-336.pdf
[iii] https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-162.pdf
[iv] Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council Text with EEA relevance, EUR-Lex – 32012R0231 – EN – EUR-Lex (europa.eu) (EUR-Lex – 32012R0231 – EN – EUR-Lex (europa.eu),
[v] https://www.fao.org/fileadmin/user_upload/jecfa_additives/docs/Monograph1/Additive-480.pdf
[vi] STANDARD of 12 July 2002 on food additives for use in foodstuffs for human consumption) Published in the Official Journal No 722a of 3 October 2002
https://legislatie.just.ro/Public/DetaliiDocumentAfis/44789
[vii] https://eur-lex.europa.eu/legal-content/RO/TXT/PDF/?uri=CELEX:02008R1333-20160525&from=FR
[viii] https://www.scridoc.com/2020/02/e340-fosfati-de-potasiu-in-ce-alimente_74.html
[ix] Re-evaluation of food additives: call for data (15.2.2012). E 340 Potassium phosphates. Submitted to EFSA on August 2012.
[x] Re-evaluation of phosphoric acid-phosphates – di-, tri- and polyphosphates (E 338-341, E 343, E 450-452) as food additives and the safety of proposed extension of use, https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2019.5674
[xi] COMMISSION REGULATION (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives https://eur-lex.europa.eu/legal-content/RO/TXT/?uri=celex%3A32011R1129

