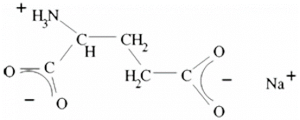
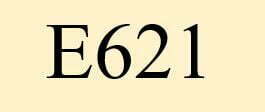What is E 621?
The food additive E621 monosodium glutamate, is a flavour enhancer. It has the synonym glutarom.
Flavour enhancers are compounds that do not have a flavour of their own but have synergistic properties with other flavourings, increasing or intensifying their flavouring power. The taste of flavour enhancers is defined by the term umami, a characteristic taste different from the four traditional tastes: sweet, salty, bitter and sour. Umami cannot be composed of the classic basic tastes, which is essential to describe how the taste sensation is conveyed.
Monosodium glutamate is the most widely used flavour enhancer.[i]
Where is it found and how is E 621 obtained?
Monosodium glutamate (MSG) is widespread in nature in free or bound form.
In the human body 20% of the protein mass is glutamate. In its free state it is found in liver, kidney, brain, blood, muscle. Monosodium glutamate is a permanent constituent of some foods: milk, eggs, meat, peas, corn, spinach, tomatoes, etc.
Monosodium glutamate comes in two forms:
– anhydrous with crude formula C5 H8 NNaO4 and molecular mass M=169.12 and
– hydrated with crude formula C5 H8 NNaO4 .H2 O and molecular mass M=187,13
Industrial is obtained by semi-synthesis, chemical synthesis and biosynthesis.
In semi-synthesis methods, the glutamic acid obtained in aqueous solution is treated with sodium hydroxide, filtered, concentrated under vacuum, cooled, and the sodium glutamate crystals are purified by recrystallisation. [ii]
The industrial production of MSG by acid hydrolysis of proteins developed steadily until 1965, the raw materials being wheat gluten, defatted soya flakes, Konbu seaweed, residues from beet sugar manufacture.
The production process consists of hydrolysis of the raw materials by heating with hydrochloric acid followed by vacuum concentration of the reaction mass until the glutamic acid hydrochloride precipitates. Purification is by dissolution in warm water, filtration and precipitation at the isoelectric point (pH = 3,2) by addition of sodium hydroxide or ammonia. The monosodium salt is obtained by treating the acid suspended in water with sodium hydroxide, decolorising with charcoal and concentrating.
Protein hydrolysis can also take place in alkaline or enzymatic media. Modern methods of MSG synthesis are fermentative processes in the presence of Micrococcus glutamicus bacteria. The raw materials are molasses or starch hydrolysates, the culture medium containing ammonium and urea salts as a source of nitrogen and biotin for bacterial cell growth. During fermentation the pH drops and is therefore corrected with gaseous ammonia. The yield of the method is about 60%. After completion of fermentation, the glutamic acid is precipitated by acidification at the isoelectric point and converted to monosodium salt by the process described above. [iii]
Which foods contain the food additive E 621?
Sodium monoglutamate (E621) can be found in the following products:
- Pate;
- Mezeluri;
- Semi-prepared;
- Juices;
- Frozen food;
- Suck the envelope;
- Mezeluri;
- Different types of sauces;
- Milk preparations;
- Frozen vegetables (including mushrooms, roots and tubers, pulses and aloe vera), seaweed, nuts and seeds;
- Fermented vegetables (including mushrooms, roots and tubers, pulses and aloe vera) and seaweed products, excluding fermented soybeans;
- Dried pasta, noodles and similar products
- Fresh meat, chicken and game;
- Frozen fish, fish fillets and fish products, including molluscs, crustaceans
- Fish in frozen batter;
- Mezeluri;
- Coffee, coffee substitutes, tea, herbal infusions;
- Chinese cooked food
Are there any side effects from consuming the food additive E 621?
There are generally no side effects, and the additive is considered safe by the Joint Expert Committee of the World Health Organisation and the Food and Agriculture Organisation.
But some people experience numbness, weakness, tremors, dizziness, headaches and palpitations or even breathing problems, panic attacks, sudden mood swings, hyperactivity, nausea, etc.
The re-evaluation of E 621 concluded that the additive did not raise genotoxicity concerns. From a neurodevelopmental toxicity study, a level at which no adverse effects were observed of 3200 mg MSG/kg body/per day could be identified. Based on the NOAEL of 3200 mg MSG/kg body/day from the neurodevelopmental toxicity study and applying the uncertainty factor of 100, an acceptable daily intake (ADI) of 30 mg/kg body/day expressed as glutamic acid was determined for glutamic acid and glutamates (E 620-625).
The EFSA Panel on food additives and nutrient sources added to food noted that DZA is below the doses that have been associated with MSG in humans: complex symptoms (> 42.9 mg/kg body weight/day), headache (85.8 mg/kg body weight/day), increased blood pressure (150 mg/kg body weight/day) and, increased insulin (> 143 mg/kg body weight/day).[iv]
What are the characteristics of E 621 sodium monoglutamate?
- Maximum daily amount allowed/body: Daily dose is not limited.
- Description of maximum daily amount allowed: The daily dose is not limited.
- Intake dose in food: 0.01
- Description of the dose for incorporation into food: It can be added to any food, including alcoholic or non-alcoholic beverages in a proportion of up to 1%, thus reducing the amount of salt required. A higher proportion than 1% will not be able to accentuate the taste any more, but may distort it.
- Side effects: NO
- Contraindications: yes
- Contraindications description:Some Japanese researchers link excessive and prolonged glutamate consumption with some degenerative brain and eye diseases.
- Usable for children: NO
- Description of use children: Not permitted in foods intended for infants and young children.
There is no acceptable daily intake set for glutamic acid and glutamates as food additives in the EU, but there is a maximum permitted level of 10 g per kg of food.
The table below shows the specifications set for monosodium glutamate by Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council.
| Chemical name L-glutamate monosodium monohydrate | E 621 MONOSODIUM GLUTAMATE
Synonyms Sodium glutamate; MSG |
| Composition Contains | not less than 99,0 % and not more than 101,0 % on the anhydrous basis |
| Solubility | Freely soluble in water; practically insoluble in ethanol or ether |
| Description | White crystals or crystalline powder, practically odourless |
| Identify | Sodium test Positive test |
| Purity | Loss on drying Not more than 0,5 % (98 °C, 5 hours) |
| Chloride | Not more than 0,2 |
| Pyrolidonecarboxylic acid | Not more than 0,2 |
| Plumb | Not more than 1 mg/kg |
Short history of sodium monoglutamate – E621
Sodium monoglutamate is one of the most common amino acids found in nature and is found in abundant amounts. In the heterogeneous group of foods it is recognised as a flavour enhancer and is used as a food additive in the form of hydrolysed protein or as a purified monosodium salt.
Sodium monoglutamate was first discovered in 1908 in Japan by examining seaweed. At that time, the extraction of sodium monoglutamate was slow and very expensive.
This food additive was first introduced in the United States in 1940. Later, in 1956, another method of large-scale production was discovered using the fermentation technique. A year later, in the United States, sodium monoglutamate was extracted by bacterial fermentation, i.e. by genetically modifying bacteria that secrete glutamic acid through their cell walls.
By 1960, sodium monoglutamate had become common among citizens, and hydrolysed protein products such as vegetable proteins and yeast were also becoming extremely popular. In the last 30 years, the use of sodium monoglutamate has increased considerably. Sodium monoglutamate is now found in frozen foods, biscuits, canned tuna, soups, processed meats, dietary supplements, salad dressings and even cosmetics.
What are food additives?
According to the WHO (World Health Organisation), substances that are added to food to maintain or improve the safety, freshness, taste, texture or appearance of food are known as food additives. For centuries, food additives have been used to preserve food, for example salt (in meat, bacon or dried fish), sugar (in marmalade) or sulphur dioxide (in wine).
Over the years, many food additives have been developed to meet the needs of food production, because large-scale food manufacturing is much more complex than small-scale home production.
The introduction of additives into food is done with the aim of ensuring that processed foods remain safe and in good condition throughout their journey from factories or industrial kitchens to warehouses and shops and ultimately to consumers.
The use of food additives is only justified when their use has a technological need, does not mislead consumers and serves a well-defined technological function such as preserving the nutritional quality of food or enhancing the stability of food.
Food additives can be derived from plants, animals or minerals, or they can be synthetic. They are intentionally added to food to fulfil certain technological purposes. There are several thousand food additives in use, all of which are designed to perform a specific task, usually to make food more durable or appealing.[v]
How are food additives assessed for risk?
The World Health Organization, in cooperation with the Food and Agriculture Organization of the United Nations (FAO), are responsible for carrying out the risk assessment of food additives. The risk assessment of food additives is carried out by an international scientific panel of experts.
Only food additives that have undergone a JECFA safety assessment and do not pose a health risk to consumers may be used. This applies regardless of whether the food additives come from a natural source or are synthetic. JECFA evaluations are based on scientific analysis of all relevant biochemical, toxicological and other data on a particular additive.
National authorities, either on the basis of the JECFA assessment or on the basis of a national assessment, may then authorise the use of food additives.
The starting point for determining whether a food additive can be used without harm is to establish the acceptable daily intake. The recommended daily intake is an estimate of the amount of additive in food or drinking water that can be safely consumed daily over a lifetime without adverse health effects.5,[vi]
Conclusions and Legislative Regulations – E 621
Sodium monoglutamate (E621) is a food additive that can be found in a variety of food products. E621 does not generally cause side effects, but some people have experienced feelings of numbness, weakness or tremor.
Sodium monoglutamate (E621) is regulated under European legislation[vii] and Commission Regulation (EU) No 231/2012. [viii]
Author: dr.ing. Ancuta Fulvia Manolache
Bibliographical references
[i] Raluca Stan, Natural and synthetic food additives, Printech Publishing House, 2007, p.84
[ii] Elena Orănescu, Food Additives-necessity and risk, SemnE Publishing House, 2005, Bucharest, 293-295
[iii] Raluca Stan, Natural and synthetic food additives, Printech Publishing House, 2007,
[iv] https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2017.4910
[v] https://www.who.int/news-room/fact-sheets/detail/food-additives
[vi] https://www.fao.org/home/en
[vii]https://webgate.ec.europa.eu/foods_system/main/?event=legislations.search&legislations.pagination=1
[viii] https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32012R0231
9 https://pixabay.com/ro/photos/chinez%c4%83-coreean%c4%83-alimente-3855829/
10 https://pixabay.com/ro/photos/platou-de-mezeluri-mezeluri-c%c3%a2rnat-668676/