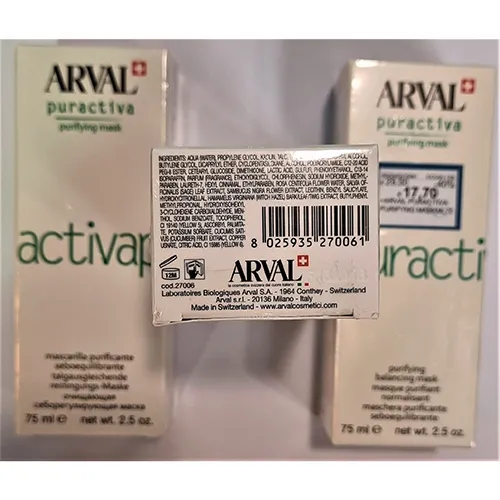
Arval – Face cream
Name: Activap
Category: 10
Date: 2024-11-22
Product Category: Cosmetics
Risk Type: Chemical
Danger: According to the list of ingredients, the product contains, 2-(4-tert-butylbenzyl) propionaldehyde (BMHCA) and hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC), substances which are prohibited in cosmetic products. BMHCA may harm the reproductive system, may harm the health of the unborn child and may cause skin sensitisation, while HICC may cause an allergic skin reaction. The product does not comply with the Cosmetic Products Regulation.
Measures: Type of economic operator to whom the measure(s) were ordered: DistributorCategory of measure(s): Ban on the marketing of the product and any accompanying measuresDate of entry into force: 10/07/2023
Description: Face cream
Notifying Country: Italy
Country of Origin: Switzerland
Alert Type: Consumer
Alert Level: Serious risk